Stoichiometry Worksheet 1 Mass Mass
This is a comprehensive, end-of-chapter fix of practice problems on stoichiometry that covers balancing chemic equations, mole-ratio calculations, limiting reactants, and pct yield concepts.
The links to the corresponding topics are given below.
- The Mole and Molar Mass
- Molar Calculations
- Per centum Limerick and Empirical Formula
- Stoichiometry of Chemical Reactions
- Limiting Reactant
- Reaction/Percent Yield
- Stoichiometry Exercise Issues
Practice
1.
Balance the following chemical equations:
a) HCl + O2 → HtwoO + Cl2
b) Al(NO3)3 + NaOH → Al(OH)3 + NaNOiii
c) H2 + N2 → NH3
d) PCl5 + HiiO → H3POfour + HCl
east) Fe + H2SOfour → Fe2(SO4)3 + Hii
f) CaCl2 + HNO3 → Ca(NO3)2 + HCl
yard) KO2 + HiiO → KOH + O2 + H2O2
h) Al + H2O → Al2O3 + H2
i) Fe + Brtwo → FeBr3
j) Cu + HNOthree → Cu(NO3)2 + NO2 + H2O
m) Al(OH)3 → Al2O3 + H2O
l) NH3 + O2 → NO + HtwoO
one thousand) Ca(AlO2)2 + HCl → AlCl3 + CaCl2 + H2O
n) C5H12 + Oii → CO2 + HtwoO
o) PivO10 + H2O → H3PO4
p) NatwoCrO4 + Pb(NO3)two → PbCrO4 + NaNO3
q) MgCl2 + AgNO3 → AgCl + Mg(NO3)2
r) KClO3 → KClO4 + KCl
due south) Ca(OH)2 + H3PO4 → Cathree(POiv)2 + H2O
a)
answer
2HCl + O2 → HiiO + Cl2
b)
reply
Al(NOiii)iii + 3NaOH → Al(OH)3 + 3NaNO3
c)
respond
3Hii + Northii → 2NH3
d)
reply
PClv + 4HiiO → H3PO4 + 5HCl
e)
respond
2Fe + 3HiiSO4 → Iron2(And so4)iii + 3Hii
f)
answer
CaClii + 2HNOiii → Ca(NO3)2 + 2HCl
thou)
answer
2KO2 + 2HiiO → 2KOH + O2 + H2O2
h)
answer
2Al + 3H2O → AliiO3 + 3H2
i)
respond
2Fe + 3Brtwo → 2FeBriii
j)
answer
Cu + 4HNOiii → Cu(NO3)2 + 2NO2 + 2HiiO
1000)
respond
2Al(OH)three → Al2O3 + 3H2O
fifty)
respond
4NHiii + 5O2 → 4NO + 6H2O
k)
answer
Ca(AlOii)2 + 8HCl → 2AlCl3 + CaCl2 + 4H2O
n)
reply
C5H12 + 8 O2 → 5CO2 + 6H2O
o)
reply
P4Ox + 6H2O → 4H3POiv
p)
answer
NatwoCrO4 + Pb(NO3)2 → PbCrO4 + 2NaNO3
q)
answer
MgCl2 + 2AgNO3 → 2AgCl + Mg(NO3)2
r)
answer
4KClO3 → 3KClO4 + KCl
south)
answer
3Ca(OH)2 + 2HthreePO4 → Caiii(POfour)2 + 6H2O
2.
Consider the counterbalanced equation:
C5H12 + 8 O2 → 5CO2 + 6HtwoO
Complete the table showing the appropriate number of moles of reactants and products.
| mol CvH12 | mol Otwo | mol COii | mol HtwoO |
| two | |||
| 2.5 | |||
| 3 | |||
| 5.4 |
answer
CfiveH12 + 8 O2 → 5COii + 6H2O
| mol C5H12 | mol Oii | mol CO2 | mol H2O |
| 2 | 16 | 10 | 12 |
| 0.3125 | two.5 | 1.5625 | 1.875 |
| 0.half dozen | 4.8 | 3 | 3.6 |
| 0.9 | 7.2 | 4.5 | 5.4 |
Solution
The moles of other molecules are calculated based on the stoichiometric ratio. For the start row the moles are calculated as follows:
\[{\rm{n}}\left( {{{\rm{O}}_{\rm{2}}}} \right)\;{\rm{ = }}\;{\rm{two }}\cancel{{{\rm{mol}}\;{{\rm{C}}_{\rm{5}}}{{\rm{H}}_{{\rm{12}}}}}}\;{\rm{ \times }}\;\frac{{{\rm{8}}\;{\rm{mol}}\;{{\rm{O}}_{\rm{two}}}}}{{{\rm{i}}\;\cancel{{{\rm{mol}}\;{{\rm{C}}_{\rm{5}}}{{\rm{H}}_{{\rm{12}}}}}}}}\;{\rm{ = }}\;{\rm{sixteen}}\;{\rm{mol}}\]
\[{\rm{n}}\left( {{\rm{C}}{{\rm{O}}_{\rm{2}}}} \right)\;{\rm{ = }}\;{\rm{2 }}\cancel{{{\rm{mol}}\;{{\rm{C}}_{\rm{five}}}{{\rm{H}}_{{\rm{12}}}}}}\;{\rm{ \times }}\;\frac{{{\rm{v}}\;{\rm{mol}}\;C{{\rm{O}}_{\rm{ii}}}}}{{{\rm{1}}\;\cancel{{{\rm{mol}}\;{{\rm{C}}_{\rm{5}}}{{\rm{H}}_{{\rm{12}}}}}}}}\;{\rm{ = }}\;{\rm{10}}\;{\rm{mol}}\]
\[{\rm{northward}}\left( {{{\rm{H}}_{\rm{2}}}{\rm{O}}} \right)\;{\rm{ = }}\;{\rm{ii }}\cancel{{{\rm{mol}}\;{{\rm{C}}_{\rm{five}}}{{\rm{H}}_{{\rm{12}}}}}}\;{\rm{ \times }}\;\frac{{{\rm{6}}\;{\rm{mol}}\;{{\rm{H}}_{\rm{2}}}{\rm{O}}}}{{{\rm{one}}\;\abolish{{{\rm{mol}}\;{{\rm{C}}_{\rm{5}}}{{\rm{H}}_{{\rm{12}}}}}}}}\;{\rm{ = }}\;{\rm{12}}\;{\rm{mol}}\]
For the 2d row :
\[{\rm{north}}\left( {{{\rm{C}}_{\rm{5}}}{{\rm{H}}_{{\rm{12}}}}} \right)\;{\rm{ = }}\;{\rm{2}}{\rm{.five }}\abolish{{{\rm{mol}}\;{{\rm{O}}_{\rm{two}}}}}\;{\rm{ \times }}\;\frac{{{\rm{ane}}\;{\rm{mol}}\;{{\rm{C}}_{\rm{5}}}{{\rm{H}}_{{\rm{12}}}}}}{{viii\;\abolish{{{\rm{mol}}\;{{\rm{O}}_{\rm{2}}}}}}}\;{\rm{ = }}\;{\rm{0}}{\rm{.3125}}\;{\rm{mol}}\]
\[{\rm{due north}}\left( {{\rm{C}}{{\rm{O}}_{\rm{2}}}} \right)\;{\rm{ = }}\;{\rm{2}}{\rm{.5 }}\cancel{{{\rm{mol}}\;{{\rm{O}}_{\rm{2}}}}}\;{\rm{ \times }}\;\frac{{{\rm{5}}\;{\rm{mol}}\;{{\rm{C}}_{\rm{5}}}{{\rm{H}}_{{\rm{12}}}}}}{{{\rm{8}}\;\abolish{{{\rm{mol}}\;{{\rm{O}}_{\rm{2}}}}}}}\;{\rm{ = }}\;{\rm{1}}{\rm{.5625}}\;{\rm{mol}}\]
\[{\rm{northward}}\left( {{{\rm{H}}_{\rm{2}}}{\rm{O}}} \right)\;{\rm{ = }}\;{\rm{two}}{\rm{.5 }}\cancel{{{\rm{mol}}\;{{\rm{O}}_{\rm{2}}}}}\;{\rm{ \times }}\;\frac{{{\rm{half-dozen}}\;{\rm{mol}}\;{{\rm{H}}_{\rm{ii}}}{\rm{O}}}}{{{\rm{viii}}\;\abolish{{{\rm{mol}}\;{{\rm{O}}_{\rm{2}}}}}}}\;{\rm{ = }}\;{\rm{1}}{\rm{.875}}\;{\rm{mol}}\]
For the third row :
\[{\rm{due north}}\left( {{{\rm{C}}_{\rm{5}}}{{\rm{H}}_{{\rm{12}}}}} \right)\;{\rm{ = }}\;{\rm{3 }}\cancel{{{\rm{mol}}\;{\rm{C}}{{\rm{O}}_{\rm{two}}}}}\;{\rm{ \times }}\;\frac{{{\rm{ane}}\;{\rm{mol}}\;{{\rm{C}}_{\rm{5}}}{{\rm{H}}_{{\rm{12}}}}}}{{{\rm{v}}\;\cancel{{{\rm{mol}}\;{\rm{C}}{{\rm{O}}_{\rm{2}}}}}}}\;{\rm{ = }}\;{\rm{0}}{\rm{.6}}\;{\rm{mol}}\]
\[{\rm{due north}}\left( {{{\rm{O}}_{\rm{2}}}} \right)\;{\rm{ = }}\;{\rm{iii }}\abolish{{{\rm{mol}}\;{\rm{C}}{{\rm{O}}_{\rm{2}}}}}\;{\rm{ \times }}\;\frac{{{\rm{8}}\;{\rm{mol}}\;{{\rm{O}}_{\rm{2}}}}}{{{\rm{5}}\;\cancel{{{\rm{mol}}\;{\rm{C}}{{\rm{O}}_{\rm{2}}}}}}}\;{\rm{ = }}\;{\rm{4}}{\rm{.viii}}\;{\rm{mol}}\]
\[{\rm{due north}}\left( {{{\rm{H}}_{\rm{2}}}{\rm{O}}} \correct)\;{\rm{ = }}\;{\rm{3 }}\cancel{{{\rm{mol}}\;{\rm{C}}{{\rm{O}}_{\rm{two}}}}}\;{\rm{ \times }}\;\frac{{{\rm{vi}}\;{\rm{mol}}\;{{\rm{H}}_{\rm{2}}}{\rm{O}}}}{{{\rm{5}}\;\cancel{{{\rm{mol}}\;{\rm{C}}{{\rm{O}}_{\rm{2}}}}}}}\;{\rm{ = }}\;{\rm{3}}{\rm{.6}}\;{\rm{mol}}\]
For the fourth row:
\[{\rm{n}}\left( {{{\rm{C}}_{\rm{5}}}{{\rm{H}}_{{\rm{12}}}}} \right)\;{\rm{ = }}\;{\rm{5}}{\rm{.iv }}\abolish{{{\rm{mol}}\;{{\rm{H}}_{\rm{2}}}{\rm{O}}}}\;{\rm{ \times }}\;\frac{{{\rm{1}}\;{\rm{mol}}\;{{\rm{C}}_{\rm{v}}}{{\rm{H}}_{{\rm{12}}}}}}{{{\rm{6}}\;\cancel{{{\rm{mol}}\;{{\rm{H}}_{\rm{2}}}{\rm{O}}}}}}\;{\rm{ = }}\;{\rm{0}}{\rm{.9}}\;{\rm{mol}}\]
\[{\rm{north}}\left( {{{\rm{O}}_{\rm{two}}}} \right)\;{\rm{ = }}\;{\rm{5}}{\rm{.four }}\abolish{{{\rm{mol}}\;{{\rm{H}}_{\rm{2}}}{\rm{O}}}}\;{\rm{ \times }}\;\frac{{{\rm{eight}}\;{\rm{mol}}\;{{\rm{O}}_{\rm{2}}}}}{{{\rm{6}}\;\abolish{{{\rm{mol}}\;{{\rm{H}}_{\rm{2}}}{\rm{O}}}}}}\;{\rm{ = }}\;{\rm{7}}{\rm{.2}}\;{\rm{mol}}\]
\[{\rm{n}}\left( {{\rm{C}}{{\rm{O}}_{\rm{ii}}}} \right)\;{\rm{ = }}\;{\rm{5}}{\rm{.4 }}\cancel{{{\rm{mol}}\;{{\rm{H}}_{\rm{2}}}{\rm{O}}}}\;{\rm{ \times }}\;\frac{{{\rm{v}}\;{\rm{mol}}\;{\rm{C}}{{\rm{O}}_{\rm{2}}}}}{{{\rm{6}}\;\cancel{{{\rm{mol}}\;{{\rm{H}}_{\rm{two}}}{\rm{O}}}}}}\;{\rm{ = }}\;{\rm{iv}}{\rm{.5}}\;{\rm{mol}}\]
3.
How many grams of COii and H2O are produced from the combustion of 220. 1000 of propane (C3H8)?
C3Hviii(k) + 5O2(yard) → 3CO2(g) + 4H2O(thou)
respond
Solution
The corporeality of production, for a given amount of reactant, is calculated based on their molar/stoichiometric ratio. Then, the first pace is to decide the moles of propane. The overall plan is:
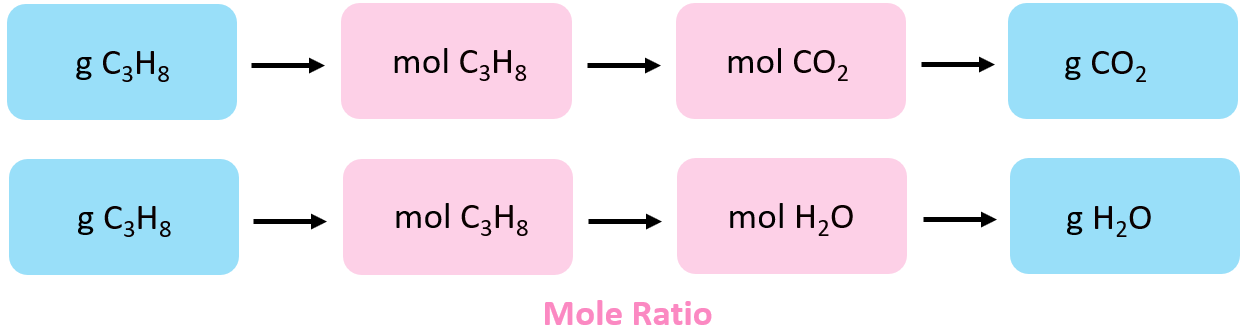
The moles of C3H8 are calculated using its molar mass:
\[{\rm{n(}}{{\rm{C}}_{\rm{3}}}{{\rm{H}}_{\rm{viii}}}{\rm{)}}\;{\rm{ = }}\;{\rm{220}}{\rm{.5}}\;{\rm{g}}\;{\rm{ \times }}\;\frac{{{\rm{1}}\;{\rm{mol}}}}{{{\rm{44}}{\rm{.1}}\;{\rm{g}}}}\;{\rm{ = }}\;{\rm{5}}{\rm{.00}}\;{\rm{mol}}\]
Next, we find the moles of CO2 based on its molar ratio with C3Height:
\[{\rm{n}}\left( {{\rm{C}}{{\rm{O}}_{\rm{2}}}} \correct)\;{\rm{ = }}\;{\rm{5}}{\rm{.00 }}\cancel{{{\rm{mol}}\;{{\rm{C}}_{\rm{3}}}{{\rm{H}}_{\rm{8}}}}}\;{\rm{ \times }}\;\frac{{{\rm{3}}\;{\rm{mol}}\;{\rm{C}}{{\rm{O}}_{\rm{2}}}}}{{{\rm{1}}\;\cancel{{{\rm{mol}}\;{{\rm{C}}_{\rm{3}}}{{\rm{H}}_{\rm{viii}}}}}}}\;{\rm{ = }}\;{\rm{15}}{\rm{.0}}\;{\rm{mol}}\]
And finally, the mass of the CO2 is adamant using its moles and molar mass:
\[{\rm{thousand}}\left( {{\rm{C}}{{\rm{O}}_{\rm{2}}}} \right)\;{\rm{ = }}\;{\rm{15}}{\rm{.0 }}\cancel{{{\rm{mol}}\;}}\;{\rm{ \times }}\;\frac{{{\rm{44}}{\rm{.0}}\;{\rm{grand}}}}{{{\rm{1}}\;\abolish{{{\rm{mol}}\;}}}}\;{\rm{ = }}\;{\rm{660}}{\rm{.}}\;{\rm{chiliad}}\]
Follow the same procedure to detect the mass of H2O:
\[{\rm{due north}}\left( {{{\rm{H}}_{\rm{two}}}{\rm{O}}} \right)\;{\rm{ = }}\;{\rm{5}}{\rm{.00 }}\abolish{{{\rm{mol}}\;{{\rm{C}}_{\rm{iii}}}{{\rm{H}}_{\rm{viii}}}}}\;{\rm{ \times }}\;\frac{{{\rm{4}}\;{\rm{mol}}\;{{\rm{H}}_{\rm{ii}}}{\rm{O}}}}{{{\rm{1}}\;\cancel{{{\rm{mol}}\;{{\rm{C}}_{\rm{iii}}}{{\rm{H}}_{\rm{8}}}}}}}\;{\rm{ = }}\;{\rm{xx}}{\rm{.0}}\;{\rm{mol}}\]
\[{\rm{m}}\left( {{{\rm{H}}_{\rm{ii}}}{\rm{O}}} \correct)\;{\rm{ = }}\;{\rm{20}}{\rm{.0 }}\abolish{{{\rm{mol}}\;}}\;{\rm{ \times }}\;\frac{{{\rm{18}}{\rm{.0}}\;{\rm{one thousand}}}}{{{\rm{ane}}\;\cancel{{{\rm{mol}}\;}}}}\;{\rm{ = }}\;{\rm{360}}{\rm{.}}\;{\rm{g}}\]
4.
How many grams of CaCltwo can be produced from 65.0 g of Ca(OH)2 according to the following reaction,
Ca(OH)2 + 2HCl → CaClii + 2HtwoO
respond
97.3 g
Solution
Here is the conceptual program for solving this trouble:
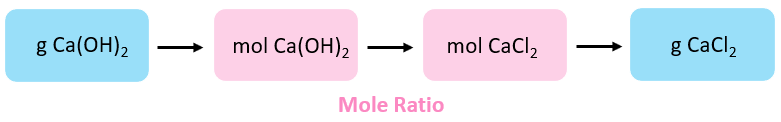
The moles of Ca(OH)two are calculated using its molar mass:
\[{\rm{due north}}\;{\rm{(Ca}}{\left( {{\rm{OH}}} \right)_{\rm{two}}}{\rm{)}}\;{\rm{ = }}\;{\rm{65}}{\rm{.0}}\;{\rm{g}}\;{\rm{ \times }}\;\frac{{{\rm{ane}}\;{\rm{mol}}}}{{{\rm{74}}{\rm{.i}}\;{\rm{g}}}}\;{\rm{ = }}\;{\rm{0}}{\rm{.877}}\;{\rm{mol}}\]
Next, nosotros find the moles of CaClii based on its molar ratio with Ca(OH)2:
\[{\rm{north}}\;\left( {{\rm{CaC}}{{\rm{l}}_{\rm{ii}}}} \correct)\;{\rm{ = }}\;{\rm{0}}{\rm{.877 }}\cancel{{{\rm{mol}}\;{\rm{Ca}}{{\left( {{\rm{OH}}} \right)}_{\rm{2}}}}}\;{\rm{ \times }}\;\frac{{{\rm{ane}}\;{\rm{mol}}\;{\rm{CaC}}{{\rm{l}}_{\rm{2}}}}}{{{\rm{1}}\;\cancel{{{\rm{mol}}\;{\rm{Ca}}{{\left( {{\rm{OH}}} \right)}_{\rm{2}}}}}}}\;{\rm{ = }}\;{\rm{0}}{\rm{.877}}\;{\rm{mol}}\]
Notice that moles of CaCl2 are equal to the moles of Ca(OH)2. This is considering of their 1:1 tooth ratio in the equation.
The terminal step is converting the moles to mass of the CaCl2:
\[{\rm{one thousand}}\left( {{\rm{CaC}}{{\rm{50}}_{\rm{2}}}} \right)\;{\rm{ = }}\;{\rm{0}}{\rm{.877 }}\cancel{{{\rm{mol}}\;}}\;{\rm{ \times }}\;\frac{{{\rm{111}}\;{\rm{yard}}}}{{{\rm{1}}\;\abolish{{{\rm{mol}}\;}}}}\;{\rm{ = }}\;{\rm{97}}{\rm{.iii}}\;{\rm{thousand}}\]
This process can, and often is shown as a 1-step conversion every bit we practise in dimensional assay. Information technology would exist as follows:
\[{\rm{m}}\left( {{\rm{CaC}}{{\rm{l}}_{\rm{2}}}} \right)\;{\rm{ = }}\;{\rm{65}}{\rm{.0}}\;{\cancel{{{\rm{g}}\;{\rm{Ca}}{{\left( {{\rm{OH}}} \right)}_{\rm{2}}}}}_{\rm{\;}}}{\rm{ \times }}\;\frac{{{\rm{1}}\;\cancel{{{\rm{mol}}\;{\rm{Ca}}{{\left( {{\rm{OH}}} \right)}_{\rm{2}}}}}}}{{{\rm{74}}{\rm{.one}}\;\cancel{{{\rm{thousand}}\;{\rm{Ca}}{{\left( {{\rm{OH}}} \correct)}_{\rm{2}}}}}}}\;{\rm{ \times }}\;\frac{{{\rm{ane}}\;\cancel{{{\rm{mol}}\;{\rm{CaC}}{{\rm{fifty}}_{\rm{two}}}}}}}{{{\rm{1}}\;\abolish{{{\rm{mol}}\;{\rm{Ca}}{{\left( {{\rm{OH}}} \right)}_{\rm{2}}}}}}}\; \times \;\frac{{{\rm{111}}\;{\rm{g}}\;{\rm{CaC}}{{\rm{50}}_{\rm{2}}}}}{{{\rm{1}}\;\cancel{{{\rm{mol}}\;{\rm{CaC}}{{\rm{l}}_{\rm{2}}}\;}}}}\;\;{\rm{ = }}\;{\rm{97}}{\rm{.3}}\;{\rm{g}}\]
This method is completely fine, still, I prefer doing these conversions stepwise to ameliorate run across what happens in each pace, and therefore, almost exercises will exist solved by doing one conversion at a fourth dimension.
5.
How many moles of oxygen are formed when 75.0 g of Cu(NO3)2 decomposes according to the following reaction?
2Cu(NOiii)2→ 2CuO + 4NO2 + O2
answer
0.200 mol
Solution
The amount of product, for a given amount of reactant, is calculated based on their molar/stoichiometric ratio.
So, the conceptual plan is:

\[{\rm{n}}\;{\rm{(Cu}}{\left( {{\rm{Northward}}{{\rm{O}}_{\rm{3}}}} \correct)_{\rm{2}}}{\rm{\;)}}\;{\rm{ = }}\;{\rm{75}}{\rm{.0}}\;{\rm{g}}\;{\rm{ \times }}\;\frac{{{\rm{one}}\;{\rm{mol}}}}{{{\rm{187}}{\rm{.5}}\;{\rm{g}}}}\;{\rm{ = }}\;{\rm{0}}{\rm{.400}}\;{\rm{mol}}\]
\[{\rm{due north}}\;\left( {{{\rm{O}}_{{\rm{2\;}}}}} \right)\;{\rm{ = }}\;{\rm{0}}{\rm{.400}}\;\cancel{{{\rm{mol}}\;{\rm{Cu}}{{\left( {{\rm{N}}{{\rm{O}}_{\rm{3}}}} \correct)}_{\rm{2}}}{\rm{\;}}}}\;{\rm{ \times }}\;\frac{{{\rm{1}}\;{\rm{mol}}\;{{\rm{O}}_{\rm{two}}}}}{{\abolish{{{\rm{2}}\;{\rm{mol}}\;{\rm{Cu}}{{\left( {{\rm{North}}{{\rm{O}}_{\rm{three}}}} \right)}_{\rm{2}}}{\rm{\;}}}}}}\;{\rm{ = }}\;{\rm{0}}{\rm{.200}}\;{\rm{mol}}\]
6.
How many grams of MnCl2can be prepared from 52.i grams of MnO2?
MnO2 + 4HCl → MnClii+ Clii + 2H2O
answer
75.v one thousand
Solution
The amount of product, for a given amount of reactant, is calculated based on their tooth/stoichiometric ratio. So, the first step is to decide the moles of propane. The overall plan is:
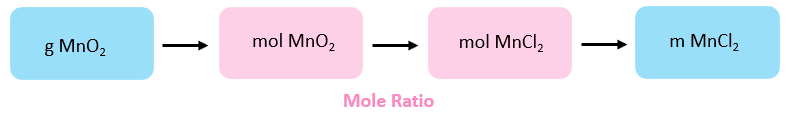
The moles of MnO2 are calculated using its molar mass:
\[{\rm{n}}\;{\rm{(Mn}}{{\rm{O}}_{\rm{ii}}}{\rm{)}}\;{\rm{ = }}\;{\rm{52}}{\rm{.1}}\;{\rm{one thousand}}\;{\rm{ \times }}\;\frac{{{\rm{i}}\;{\rm{mol}}}}{{{\rm{86}}{\rm{.9}}\;{\rm{g}}}}\;{\rm{ = }}\;{\rm{0}}{\rm{.600}}\;{\rm{mol}}\]
Next, we find the moles of MnCl2 based on its molar ratio with MnOii:
\[{\rm{n}}\;\left( {{\rm{MnC}}{{\rm{l}}_{\rm{ii}}}} \right)\;{\rm{ = }}\;{\rm{0}}{\rm{.600 }}\cancel{{{\rm{mol}}\;{\rm{Mn}}{{\rm{O}}_{\rm{ii}}}}}\;{\rm{ \times }}\;\frac{{{\rm{ane}}\;{\rm{mol}}\;{\rm{MnC}}{{\rm{l}}_{\rm{ii}}}}}{{{\rm{1}}\;\cancel{{{\rm{mol}}\;{\rm{Mn}}{{\rm{O}}_{\rm{2}}}}}}}\;{\rm{ = }}\;{\rm{0}}{\rm{.600}}\;{\rm{mol}}\]
And finally, the mass of the MnCl2 is determined using its moles and molar mass:
\[{\rm{g}}\left( {{\rm{MnC}}{{\rm{l}}_{\rm{2}}}} \right)\;{\rm{ = }}\;{\rm{0}}{\rm{.600 }}\cancel{{{\rm{mol}}\;}}\;{\rm{ \times }}\;\frac{{{\rm{125}}{\rm{.viii}}\;{\rm{g}}}}{{{\rm{1}}\;\cancel{{{\rm{mol}}\;}}}}\;{\rm{ = }}\;{\rm{75}}{\rm{.5g}}\]
7.
Determine the mass of oxygen that is formed when an 18.3-1000 sample of potassium chlorate is decomposed according to the following equation:
2KClO3(s) → 2KCl(s) + 3Oii(one thousand).
answer
7.xvi one thousand
Solution
The amount of product, for a given corporeality of reactant, is calculated based on their molar/stoichiometric ratio. And then, the first footstep is to decide the moles of propane. The conceptual plan is:
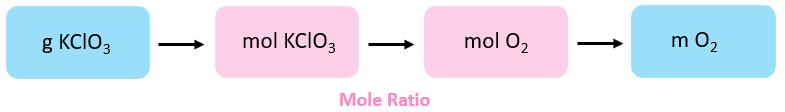
The moles of MnO2 are calculated using its molar mass:
\[{\rm{n}}\;{\rm{(KCl}}{{\rm{O}}_{\rm{3}}}{\rm{)}}\;{\rm{ = }}\;{\rm{eighteen}}{\rm{.iii}}\;{\rm{chiliad}}\;{\rm{ \times }}\;\frac{{{\rm{1}}\;{\rm{mol}}}}{{{\rm{122}}{\rm{.6}}\;{\rm{g}}}}\;{\rm{ = }}\;{\rm{0}}{\rm{.149}}\;{\rm{mol}}\]
Next, we detect the moles of O2 based on its molar ratio with KClO3:
\[{\rm{n}}\;\left( {{{\rm{O}}_{\rm{2}}}} \correct)\;{\rm{ = }}\;{\rm{0}}{\rm{.149 }}\abolish{{{\rm{mol}}\;{\rm{KCl}}{{\rm{O}}_{\rm{3}}}}}\;{\rm{ \times }}\;\frac{{{\rm{3}}\;{\rm{mol}}\;{{\rm{O}}_{\rm{2}}}}}{{{\rm{two}}\;\abolish{{{\rm{mol}}\;{\rm{KCl}}{{\rm{O}}_{\rm{3}}}}}}}\;{\rm{ = }}\;{\rm{0}}{\rm{.224}}\;{\rm{mol}}\]
And finally, the mass of the MnCltwo is determined using its moles and molar mass:
\[{\rm{m}}\left( {{{\rm{O}}_{\rm{2}}}} \right)\;{\rm{ = }}\;{\rm{0}}{\rm{.224 }}\cancel{{{\rm{mol}}\;}}\;{\rm{ \times }}\;\frac{{{\rm{32}}{\rm{.0}}\;{\rm{grand}}}}{{{\rm{1}}\;\cancel{{{\rm{mol}}\;}}}}\;{\rm{ = }}\;{\rm{7}}{\rm{.16}}\;{\rm{m}}\]
8.
How many grams of H2O will be formed when 48.0 grams H2 are mixed with excess hydrogen gas?
2H2 + Otwo → 2H2O
respond
432 one thousand
Solution
The amount of production, for a given amount of reactant, is calculated based on their molar/stoichiometric ratio. So, the offset step is to determine the moles of propane. The conceptual plan is:
The moles of HiiO are calculated using its molar mass:
\[{\rm{n}}\;{\rm{(}}{{\rm{H}}_2}{\rm{)}}\;{\rm{ = }}\;{\rm{48}}{\rm{.0}}\;{\rm{g}}\;{\rm{ \times }}\;\frac{{{\rm{1}}\;{\rm{mol}}}}{{{\rm{2}}{\rm{.00}}\;{\rm{chiliad}}}}\;{\rm{ = }}\;{\rm{24}}{\rm{.0}}\;{\rm{mol}}\]
Next, we find the moles of HtwoO based on its molar ratio with Htwo:
\[{\rm{n}}\;\left( {{{\rm{H}}_{\rm{2}}}{\rm{O}}} \right)\;{\rm{ = }}\;{\rm{24}}{\rm{.0 }}\cancel{{{\rm{mol}}\;{{\rm{H}}_{\rm{ii}}}}}\;{\rm{ \times }}\;\frac{{{\rm{ii}}\;{\rm{mol}}\;{{\rm{H}}_{\rm{2}}}{\rm{O}}}}{{{\rm{two}}\;\cancel{{{\rm{mol}}\;{{\rm{H}}_{\rm{2}}}}}}}\;{\rm{ = }}\;{\rm{24}}{\rm{.0}}\;{\rm{mol}}\]
And finally, the mass of the H2O is determined using its moles and molar mass:
\[{\rm{m}}\left( {{{\rm{H}}_{\rm{2}}}{\rm{O}}} \right)\;{\rm{ = }}\;{\rm{24}}{\rm{.0 }}\cancel{{{\rm{mol}}\;}}\;{\rm{ \times }}\;\frac{{{\rm{eighteen}}{\rm{.0}}\;{\rm{m}}}}{{{\rm{i}}\;\cancel{{{\rm{mol}}\;}}}}\;{\rm{ = }}\;{\rm{432}}\;{\rm{g}}\]
9.
Consider the chlorination reaction of methane (CH4):
CH4(g) + 4Cl2(g) → CCl4(g) + 4HCl(1000)
What mass of CCliv will be produced if 0.338 moles of CH4 are used in the reaction?
reply
0.337 mol
Solution
The amount of production, for a given amount of reactant, is calculated based on their molar/stoichiometric ratio. And then, the starting time step is to decide the moles of propane. The conceptual program is:
The moles of CCl4 are calculated using its tooth mass:
\[{\rm{n}}\;{\rm{(CC}}{{\rm{l}}_{\rm{four}}}{\rm{)}}\;{\rm{ = }}\;{\rm{51}}{\rm{.9}}\;{\rm{g}}\;{\rm{ \times }}\;\frac{{{\rm{1}}\;{\rm{mol}}}}{{{\rm{153}}{\rm{.8}}\;{\rm{g}}}}\;{\rm{ = }}\;{\rm{0}}{\rm{.337}}\;{\rm{mol}}\]
Next, we find the moles of CHiv based on its molar ratio with CCl4.It is a 1:1 ratio, and then there is going to be 0.337 mol CHfour:
\[{\rm{n}}\;\left( {{\rm{C}}{{\rm{H}}_{\rm{iv}}}} \right)\;{\rm{ = }}\;{\rm{0}}{\rm{.337 }}\cancel{{{\rm{mol}}\;{\rm{CC}}{{\rm{l}}_{\rm{4}}}}}\;{\rm{ \times }}\;\frac{{{\rm{1}}\;{\rm{mol}}\;{\rm{C}}{{\rm{H}}_{\rm{4}}}}}{{{\rm{1}}\;\abolish{{{\rm{mol}}\;{\rm{CC}}{{\rm{l}}_{\rm{4}}}}}}}\;{\rm{ = }}\;{\rm{0}}{\rm{.337}}\;{\rm{mol}}\]
ten.
How many grams of Ba(NO3)2 can be produced by reacting 16.5 m of HNOiii with an excess of Ba(OH)ii?
answer
34.ii k
Solution
Nosotros need to first write the balanced chemical equation to do the calculations:
Ba(OH)ii + 2HNOiii → Ba(NO3)2 + 2H2O
The conceptual programme would be:
So, let's first with the moles of HNO3:
\[{\rm{n}}\;{\rm{(HN}}{{\rm{O}}_{\rm{3}}}{\rm{)}}\;{\rm{ = }}\;{\rm{16}}{\rm{.five}}\;{\rm{g}}\;{\rm{ \times }}\;\frac{{{\rm{i}}\;{\rm{mol}}}}{{{\rm{63}}{\rm{.0}}\;{\rm{g}}}}\;{\rm{ = }}\;{\rm{0}}{\rm{.262}}\;{\rm{mol}}\]
Once nosotros have the moles, we can determine the moles of Ba(NO3)2 based on the molar ratio, and then convert the moles to the mass:
\[{\rm{due north}}\;\left( {{\rm{Ba}}{{\left( {{\rm{N}}{{\rm{O}}_{\rm{3}}}} \correct)}_{\rm{2}}}} \correct)\;{\rm{ = }}\;{\rm{0}}{\rm{.262 }}\abolish{{{\rm{mol}}\;{\rm{HN}}{{\rm{O}}_{\rm{3}}}}}\;{\rm{ \times }}\;\frac{{{\rm{1}}\;{\rm{mol}}\;{\rm{Ba}}{{\left( {{\rm{Northward}}{{\rm{O}}_{\rm{3}}}} \right)}_{\rm{two}}}}}{{{\rm{2}}\;\cancel{{{\rm{mol}}\;{\rm{HN}}{{\rm{O}}_{\rm{3}}}}}}}\;{\rm{ = }}\;{\rm{0}}{\rm{.131}}\;{\rm{mol}}\]
\[{\rm{m}}\left( {{\rm{Ba}}{{\left( {{\rm{North}}{{\rm{O}}_{\rm{three}}}} \correct)}_{\rm{two}}}} \correct)\;{\rm{ = }}\;{\rm{0}}{\rm{.131 }}\cancel{{{\rm{mol}}\;}}\;{\rm{ \times }}\;\frac{{{\rm{261}}{\rm{.3}}\;{\rm{g}}}}{{{\rm{ane}}\;\cancel{{{\rm{mol}}\;}}}}\;{\rm{ = }}\;{\rm{34}}{\rm{.ii}}\;{\rm{g}}\]
11.
Ethanol tin exist obtained by fermentation – a complex chemical process breaking down glucose to ethanol and carbon dioxide.
C6H12O6 → 2C2H5OH + 2COtwo
glucose ethanol
How many mL of ethanol (d =0.789 g/mL) can be obtained by this process starting with 286 m of glucose?
answer
186 mL
Solution
This problem has an additional footstep of converting the mass of the production to book. Other than that, we follow the aforementioned strategy and steps as in the previous bug:
\[{\rm{north}}\;{\rm{(}}{{\rm{C}}_{\rm{vi}}}{{\rm{H}}_{{\rm{12}}}}{{\rm{O}}_{\rm{6}}}{\rm{)}}\;{\rm{ = }}\;{\rm{286}}\;{\rm{k}}\;{\rm{ \times }}\;\frac{{{\rm{1}}\;{\rm{mol}}}}{{{\rm{180}}{\rm{.2}}\;{\rm{k}}}}\;{\rm{ = }}\;{\rm{1}}{\rm{.59}}\;{\rm{mol}}\]
At present, nosotros can find the moles of C2H5OH:
\[{\rm{n}}\;\left( {{{\rm{C}}_{\rm{ii}}}{{\rm{H}}_{\rm{five}}}{\rm{OH}}} \correct)\;{\rm{ = }}\;{\rm{one}}{\rm{.59 }}\cancel{{{\rm{mol}}\;{{\rm{C}}_{\rm{6}}}{{\rm{H}}_{{\rm{12}}}}{{\rm{O}}_{\rm{half dozen}}}}}\;{\rm{ \times }}\;\frac{{{\rm{2}}\;{\rm{mol}}\;{{\rm{C}}_{\rm{two}}}{{\rm{H}}_{\rm{5}}}{\rm{OH}}}}{{{\rm{1}}\;\abolish{{{\rm{mol}}\;{{\rm{C}}_{\rm{half dozen}}}{{\rm{H}}_{{\rm{12}}}}{{\rm{O}}_{\rm{6}}}}}}}\;{\rm{ = }}\;{\rm{3}}{\rm{.eighteen}}\;{\rm{mol}}\]
\[{\rm{m}}\left( {{{\rm{C}}_{\rm{two}}}{{\rm{H}}_{\rm{5}}}{\rm{OH}}} \right)\;{\rm{ = }}\;{\rm{three}}{\rm{.18 }}\cancel{{{\rm{mol}}\;}}\;{\rm{ \times }}\;\frac{{{\rm{46}}{\rm{.i}}\;{\rm{g}}}}{{{\rm{1}}\;\cancel{{{\rm{mol}}\;}}}}\;{\rm{ = }}\;{\rm{147}}\;{\rm{g}}\]
The terminal step is converting the mass to volume:
\[{\rm{v}}\left( {{{\rm{C}}_{\rm{2}}}{{\rm{H}}_{\rm{five}}}{\rm{OH}}} \correct)\;{\rm{ = }}\;{\rm{147}}\;\abolish{{\rm{g}}}\;{\rm{ \times }}\;\frac{{{\rm{1}}\;{\rm{mL}}}}{{{\rm{0}}{\rm{.789}}\;\cancel{{\rm{g}}}}}\;{\rm{ = }}\;{\rm{186}}\;{\rm{mL}}\]
12.
36.0 1000 of butane (C4Hten) was burned in an excess of oxygen and the resulting carbon dioxide (COii) was collected in a sealed vessel.
2C4H10 + 13Otwo → 8CO2 + 10H2O
How many grams of LiOH will be necessary to consume all the CO2 from the first reaction?
2LiOH + CO2 → Li2CO3 + H2O
answer
119 g
Solution
There are two reactions here, and the link betwixt them is the COtwo that is formed in the first reaction and used in the second.
So, the plan would be to decide the moles of CO2 in the first reaction, and use information technology to calculate the moles, and consequently the mass of LiOH.
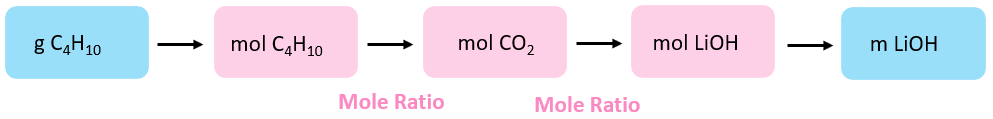
\[{\rm{n}}\;{\rm{(}}{{\rm{C}}_{\rm{iv}}}{{\rm{H}}_{{\rm{10}}}}{\rm{)}}\;{\rm{ = }}\;{\rm{36}}{\rm{.0}}\;{\rm{1000}}\;{\rm{ \times }}\;\frac{{{\rm{1}}\;{\rm{mol}}}}{{{\rm{58}}{\rm{.1}}\;{\rm{chiliad}}}}\;{\rm{ = }}\;{\rm{0}}{\rm{.620}}\;{\rm{mol}}\]
\[{\rm{n}}\;\left( {{\rm{C}}{{\rm{O}}_{\rm{2}}}} \right)\;{\rm{ = }}\;{\rm{0}}{\rm{.620 }}\cancel{{{\rm{mol}}\;{{\rm{C}}_{\rm{4}}}{{\rm{H}}_{{\rm{10}}}}}}\;{\rm{ \times }}\;\frac{{{\rm{8}}\;{\rm{mol}}\;{\rm{C}}{{\rm{O}}_{\rm{2}}}}}{{{\rm{2}}\cancel{{{\rm{mol}}\;{{\rm{C}}_{\rm{four}}}{{\rm{H}}_{{\rm{10}}}}}}}}\;{\rm{ = }}\;{\rm{2}}{\rm{.48}}\;{\rm{mol}}\]
This is the amount of COtwo that will exist reacted with LiOH in a ane:2 ratio. So, we can determine the moles of LiOH and convert them to the mass:
\[{\rm{northward}}\;\left( {{\rm{LiOH}}} \correct)\;{\rm{ = }}\;{\rm{ii}}{\rm{.48 }}\cancel{{{\rm{mol}}\;{\rm{C}}{{\rm{O}}_{\rm{2}}}}}\;{\rm{ \times }}\;\frac{{{\rm{ii}}\;{\rm{mol}}\;{\rm{LiOH}}}}{{{\rm{1}}\;\cancel{{{\rm{mol}}\;{\rm{C}}{{\rm{O}}_{\rm{2}}}}}}}\;{\rm{ = }}\;{\rm{4}}{\rm{.96}}\;{\rm{mol}}\]
\[{\rm{m}}\;\left( {{\rm{LiOH}}} \correct)\;{\rm{ = }}\;{\rm{4}}{\rm{.96}}\;\abolish{{{\rm{mol}}\;{\rm{LiOH}}}}\;{\rm{ \times }}\;\frac{{{\rm{24}}{\rm{.0}}\;{\rm{g}}\;{\rm{LiOH}}}}{{{\rm{one}}\;\abolish{{{\rm{mol}}\;{\rm{LiOH}}}}}}\;{\rm{ = }}\;{\rm{119}}\;{\rm{g}}\;\]
13.
Limiting Reactant
13. Which statement most limiting reactant is right?
a) The limiting reactant is the one in a smaller quantity.
b) The limiting reactant is the one in greater quantity.
c) The limiting reactant is the one producing less product.
d) The limiting reactant is the one producing more production.
answer
c) The limiting reactant is the ane producing less product than any of the other reactants.
14.
Find the limiting reactant for each initial amount of reactants.
4NH3 + 5O2 → 4NO + 6H2O
a) 2 mol of NHthree and 2 mol of O2
b) 2 mol of NHiii and 3 mol of O2
c) 3 mol of NH3 and 3 mol of Otwo
d) three mol of NHiii and two mol of Otwo
Notation: This is not a multiple-choice question. Each row represents a divide question where you demand to determine the limiting reactant.
answer
a) ii mol of NH3 and two mol of Otwo
b) 2 mol of NH3 and 3 mol of Oii
c) 3 mol of NH3 and 3 mol of O2
d) three mol of NH3 and 2 mol of O2
Solution
The limiting reactant is the one producing less product. So, to determine the limiting reactant for each pair, we need to option a product (NO or HiiO) and meet whether NH3 or O2 will produce less product for the given mole ratio.
Let's do the calculations based on the corporeality of NO that can be formed.
a)
\[{\rm{north}}\;\left( {{\rm{NO}}} \right)\;{\rm{ = }}\;{\rm{2 }}\cancel{{{\rm{mol}}\;{\rm{Northward}}{{\rm{H}}_{\rm{iii}}}}}\;{\rm{ \times }}\;\frac{{{\rm{4}}\;{\rm{mol}}\;{\rm{NO}}}}{{{\rm{4}}\;\cancel{{{\rm{mol}}\;{\rm{Due north}}{{\rm{H}}_{\rm{3}}}}}}}\;{\rm{ = }}\;{\rm{2}}\;{\rm{mol}}\]
\[{\rm{n}}\;\left( {{\rm{NO}}} \right)\;{\rm{ = }}\;{\rm{ii }}\cancel{{{\rm{mol}}\;{{\rm{O}}_2}}}\;{\rm{ \times }}\;\frac{{{\rm{4}}\;{\rm{mol}}\;{\rm{NO}}}}{{{\rm{5}}\;\cancel{{{\rm{mol}}\;{{\rm{O}}_2}}}}}\;{\rm{ = }}\;{\rm{i}}{\rm{.6}}\;{\rm{mol}}\]
And so, for (a), O2 is the limiting reactant.
b)
\[{\rm{n}}\;\left( {{\rm{NO}}} \right)\;{\rm{ = }}\;{\rm{2 }}\cancel{{{\rm{mol}}\;{\rm{North}}{{\rm{H}}_{\rm{3}}}}}\;{\rm{ \times }}\;\frac{{{\rm{4}}\;{\rm{mol}}\;{\rm{NO}}}}{{{\rm{iv}}\;\cancel{{{\rm{mol}}\;{\rm{N}}{{\rm{H}}_{\rm{iii}}}}}}}\;{\rm{ = }}\;{\rm{two}}\;{\rm{mol}}\]
\[{\rm{n}}\;\left( {{\rm{NO}}} \correct)\;{\rm{ = }}\;{\rm{3 }}\abolish{{{\rm{mol}}\;{{\rm{O}}_2}}}\;{\rm{ \times }}\;\frac{{{\rm{iv}}\;{\rm{mol}}\;{\rm{NO}}}}{{{\rm{5}}\;\cancel{{{\rm{mol}}\;{{\rm{O}}_2}}}}}\;{\rm{ = }}\;{\rm{2}}{\rm{.4}}\;{\rm{mol}}\]
So, for (b), NO is the limiting reactant.
c)
\[{\rm{northward}}\;\left( {{\rm{NO}}} \right)\;{\rm{ = }}\;{\rm{three }}\cancel{{{\rm{mol}}\;{\rm{Northward}}{{\rm{H}}_{\rm{3}}}}}\;{\rm{ \times }}\;\frac{{{\rm{4}}\;{\rm{mol}}\;{\rm{NO}}}}{{{\rm{4}}\;\abolish{{{\rm{mol}}\;{\rm{Due north}}{{\rm{H}}_{\rm{3}}}}}}}\;{\rm{ = }}\;{\rm{three}}\;{\rm{mol}}\]
\[{\rm{n}}\;\left( {{\rm{NO}}} \right)\;{\rm{ = }}\;{\rm{3 }}\cancel{{{\rm{mol}}\;{{\rm{O}}_2}}}\;{\rm{ \times }}\;\frac{{{\rm{four}}\;{\rm{mol}}\;{\rm{NO}}}}{{{\rm{v}}\;\abolish{{{\rm{mol}}\;{{\rm{O}}_2}}}}}\;{\rm{ = }}\;{\rm{two}}{\rm{.4}}\;{\rm{mol}}\]
So, for (c), O2 is the limiting reactant.
d)
\[{\rm{n}}\;\left( {{\rm{NO}}} \right)\;{\rm{ = }}\;{\rm{3 }}\cancel{{{\rm{mol}}\;{\rm{N}}{{\rm{H}}_{\rm{3}}}}}\;{\rm{ \times }}\;\frac{{{\rm{iv}}\;{\rm{mol}}\;{\rm{NO}}}}{{{\rm{4}}\;\cancel{{{\rm{mol}}\;{\rm{Northward}}{{\rm{H}}_{\rm{iii}}}}}}}\;{\rm{ = }}\;{\rm{three}}\;{\rm{mol}}\]
\[{\rm{n}}\;\left( {{\rm{NO}}} \correct)\;{\rm{ = }}\;{\rm{two }}\cancel{{{\rm{mol}}\;{{\rm{O}}_2}}}\;{\rm{ \times }}\;\frac{{{\rm{four}}\;{\rm{mol}}\;{\rm{NO}}}}{{{\rm{5}}\;\cancel{{{\rm{mol}}\;{{\rm{O}}_2}}}}}\;{\rm{ = }}\;{\rm{1}}{\rm{.vi}}\;{\rm{mol}}\]
So, for (d), O2 is the limiting reactant.
The shorter way of determining the limiting reactant is to divide the moles of a given reactant by its coefficient in the chemic reaction. This is the constructive quantity of the reactant as it takes into consideration not simply the amount of the reactant but as well its molar ratio in the reaction.
Let'south put the equation and the moles here and determine the LR past the ratio of the moles and coefficient:
4NH3 + 5O2 → 4NO + 6H2O
a) 2 mol of NHiii and 2 mol of O2
b) two mol of NH3 and iii mol of Otwo
c) 3 mol of NH3 and three mol of Oii
d) iii mol of NH3 and two mol of O2
So, for (a), we are comparing 2/four mol of NH3 with 2/five mol of O2 , and therefore, Oii is the LR.
For (b), we are comparing 2/4 mol of NHiii with 3/5 mol of O2 , and therefore, NHthree is the LR.
For (c), we are comparing three/4 mol of NH3 with iii/5 mol of O2 , and therefore, O2 is the LR.
For (d), 3 mol of NHthree and 3/4 mol of NH 3 with 2/5 mol of O2, and therefore, O2 is the LR.
15.
How many g of hydrogen are left over in producing ammonia when 14.0 thousand of nitrogen is reacted with viii.0 g of hydrogen?
Nii(chiliad) + 3 H2(grand) → two NH3(grand)
answer
5.0 m
Solution
When the amounts of both reactants are given, you must determine the LR and then, based on the moles of the LR, determine the amount of production(s) that tin be formed. This is the standard procedure when working on examples covering limiting reactant.
Now, in this problem, we are not asked to determine the amount of whatever product, but rather we demand to determine how much hydrogen is left out in one case the reaction is complete. This already tells us that the nitrogen is the LR considering some of the hydrogen remains unreacted, then it must've been in excess.
The beginning step is, as always, to make up one's mind the moles of the reactants, then using the mole ratio, summate how much hydrogen has reacted. The remaining mass of the hydrogen is calculated by subtracting this amount from the initial mass.
\[{\rm{northward}}\;{\rm{(}}{{\rm{Northward}}_{\rm{two}}}{\rm{)}}\;{\rm{ = }}\;{\rm{14}}{\rm{.0}}\;{\rm{g}}\;{\rm{ \times }}\;\frac{{{\rm{1}}\;{\rm{mol}}}}{{{\rm{28}}{\rm{.0}}\;{\rm{g}}}}\;{\rm{ = }}\;{\rm{0}}{\rm{.500}}\;{\rm{mol}}\]
\[{\rm{north}}\;{\rm{(}}{{\rm{H}}_{\rm{two}}}{\rm{)}}\;{\rm{ = }}\;{\rm{8}}{\rm{.0}}\;{\rm{g}}\;{\rm{ \times }}\;\frac{{{\rm{1}}\;{\rm{mol}}}}{{{\rm{2}}{\rm{.0}}\;{\rm{chiliad}}}}\;{\rm{ = }}\;{\rm{4}}{\rm{.0}}\;{\rm{mol}}\]
The moles of hydrogen reacted are:
So, there are:
4.0 – 1.50 = ii.5 mol Hii left out
\[{\rm{m}}\;{\rm{(}}{{\rm{H}}_{\rm{2}}}{\rm{)}}\;{\rm{ = }}\;{\rm{2}}{\rm{.5}}\;{\rm{mol}}\;{\rm{ \times }}\;\frac{{{\rm{2}}{\rm{.0}}\;{\rm{g}}}}{{{\rm{1}}\;{\rm{mol}}}}\;{\rm{ = }}\;{\rm{5}}{\rm{.0}}\;{\rm{g}}\]
16.
How many grams of PCliii volition be produced if 38.five g Cl2 is reacted with 56.4 g P4 according to the following equation?
6Cl2(g) + P4(s) → 4PClthree(l)
answer
169 g
Solution
When the amounts of both reactants are given, yous must determine the LR and so, based on the moles of the LR, determine the amount of product(s) that can be formed.
The moles of Cl2 and P4 are:
\[{\rm{n}}\;{\rm{(C}}{{\rm{l}}_{\rm{2}}}{\rm{)}}\;{\rm{ = }}\;{\rm{38}}{\rm{.5}}\;{\rm{thousand}}\;{\rm{ \times }}\;\frac{{{\rm{one}}\;{\rm{mol}}}}{{{\rm{70}}{\rm{.9}}\;{\rm{g}}}}\;{\rm{ = }}\;{\rm{1}}{\rm{.84}}\;{\rm{mol}}\]
\[{\rm{n}}\;{\rm{(}}{{\rm{P}}_{\rm{4}}}{\rm{)}}\;{\rm{ = }}\;{\rm{56}}{\rm{.iv}}\;{\rm{g}}\;{\rm{ \times }}\;\frac{{{\rm{ane}}\;{\rm{mol}}}}{{{\rm{123}}{\rm{.9}}\;{\rm{thousand}}}}\;{\rm{ = }}\;{\rm{0}}{\rm{.455}}\;{\rm{mol}}\]
Next, nosotros calculate how much PCl3 can be formed from each reactant:
\[{\rm{n}}\;\left( {{\rm{PC}}{{\rm{fifty}}_{\rm{three}}}} \right)\;{\rm{ = }}\;{\rm{1}}{\rm{.84 }}\cancel{{{\rm{mol}}\;{\rm{C}}{{\rm{50}}_{\rm{two}}}}}\;{\rm{ \times }}\;\frac{{{\rm{iv}}\;{\rm{mol}}\;{\rm{PC}}{{\rm{l}}_{\rm{three}}}}}{{{\rm{6}}\;\cancel{{{\rm{mol}}\;{\rm{C}}{{\rm{fifty}}_{\rm{2}}}}}}}\;{\rm{ = }}\;{\rm{1}}{\rm{.23}}\;{\rm{mol}}\]
\[{\rm{northward}}\;\left( {{\rm{PC}}{{\rm{fifty}}_{\rm{3}}}} \right)\;{\rm{ = }}\;{\rm{0}}{\rm{.455 }}\cancel{{{\rm{mol}}\;{{\rm{P}}_{\rm{iv}}}}}\;{\rm{ \times }}\;\frac{{{\rm{4}}\;{\rm{mol}}\;{\rm{PC}}{{\rm{fifty}}_{\rm{3}}}}}{{{\rm{1}}\;\cancel{{{\rm{mol}}\;{{\rm{P}}_{\rm{4}}}}}}}\;{\rm{ = }}\;{\rm{1}}{\rm{.82}}\;{\rm{mol}}\]
Clii gives less PCl3 and therefore, it is the LR and 1.23 mol of PCliii can be produced in this reaction.
The mass of PCl3 is:
\[{\rm{m}}\;{\rm{(PC}}{{\rm{l}}_{\rm{3}}}{\rm{)}}\;{\rm{ = }}\;{\rm{1}}{\rm{.23}}\;{\rm{mol}}\;{\rm{ \times }}\;\frac{{{\rm{137}}{\rm{.3}}\;{\rm{g}}}}{{{\rm{1}}\;{\rm{mol}}}}\;{\rm{ = }}\;{\rm{169}}\;{\rm{grand}}\]
17.
How many grams of sulfur can be obtained if 12.half-dozen one thousand HiiS is reacted with 14.6 g SOtwo according to the following equation?
2H2Southward(thou) + Thentwo(thou) → 3S(s) + 2HtwoO(g)
answer
17.eight g
Solution
When the amount of both reactants is given, you must make up one's mind the LR then, based on the moles of the LR, make up one's mind the corporeality of production(south) that can be formed.
The moles of HtwoS and Sotwo are:
\[{\rm{due north}}\;{\rm{(}}{{\rm{H}}_{\rm{2}}}{\rm{Due south)}}\;{\rm{ = }}\;{\rm{12}}{\rm{.vi}}\;{\rm{g}}\;{\rm{ \times }}\;\frac{{{\rm{1}}\;{\rm{mol}}}}{{{\rm{34}}{\rm{.one}}\;{\rm{one thousand}}}}\;{\rm{ = }}\;{\rm{0}}{\rm{.370}}\;{\rm{mol}}\]
\[{\rm{northward}}\;{\rm{(S}}{{\rm{O}}_{\rm{2}}}{\rm{)}}\;{\rm{ = }}\;{\rm{14}}{\rm{.half-dozen}}\;{\rm{one thousand}}\;{\rm{ \times }}\;\frac{{{\rm{1}}\;{\rm{mol}}}}{{{\rm{64}}{\rm{.i}}\;{\rm{g}}}}\;{\rm{ = }}\;{\rm{0}}{\rm{.228}}\;{\rm{mol}}\]
Next, we calculate how much PCl3 can exist formed from each reactant:
\[{\rm{n}}\;\left( {\rm{S}} \correct)\;{\rm{ = }}\;{\rm{0}}{\rm{.370 }}\cancel{{{\rm{mol}}\;{{\rm{H}}_{\rm{2}}}{\rm{S}}}}\;{\rm{ \times }}\;\frac{{{\rm{3}}\;{\rm{mol}}\;{\rm{S}}}}{{{\rm{2}}\;\abolish{{{\rm{mol}}\;{{\rm{H}}_{\rm{2}}}{\rm{S}}}}}}\;{\rm{ = }}\;{\rm{0}}{\rm{.555}}\;{\rm{mol}}\]
\[{\rm{n}}\;\left( {\rm{Southward}} \right)\;{\rm{ = }}\;{\rm{0}}{\rm{.228 }}\cancel{{{\rm{mol}}\;{\rm{S}}{{\rm{O}}_{\rm{2}}}}}\;{\rm{ \times }}\;\frac{{{\rm{3}}\;{\rm{mol}}\;{\rm{Due south}}}}{{{\rm{ane}}\;\abolish{{{\rm{mol}}\;{\rm{S}}{{\rm{O}}_{\rm{2}}}}}}}\;{\rm{ = }}\;{\rm{0}}{\rm{.684}}\;{\rm{mol}}\]
HiiS gives less Due south and therefore, it is the LR and 0.555 mol of S can be produced in this reaction.
The mass of S is:
\[{\rm{m}}\;{\rm{(S)}}\;{\rm{ = }}\;{\rm{0}}{\rm{.555}}\;{\rm{mol}}\;{\rm{ \times }}\;\frac{{{\rm{32}}{\rm{.1}}\;{\rm{g}}}}{{{\rm{i}}\;{\rm{mol}}}}\;{\rm{ = }}\;{\rm{17}}{\rm{.eight}}\;{\rm{yard}}\]
18.
The post-obit equation represents the combustion of octane, C8H18, a component of gasoline:
2C8H18(yard) + 25Otwo(g) → 16COii(one thousand) + 18H2O(yard)
Will 356 thou of oxygen be enough for the complete combustion of 954 g of octane?
answer
356 yard of oxygen is not enough for the complete combustion of 954 thou of octane.
Solution
First, calculate the moles of the reactants:
\[{\rm{n}}\;{\rm{(}}{{\rm{O}}_{\rm{ii}}}{\rm{)}}\;{\rm{ = }}\;{\rm{356}}\;{\rm{m}}\;{\rm{ \times }}\;\frac{{{\rm{one}}\;{\rm{mol}}}}{{{\rm{32}}{\rm{.0}}\;{\rm{g}}}}\;{\rm{ = }}\;{\rm{eleven}}{\rm{.4}}\;{\rm{mol}}\]
\[{\rm{n}}\;{\rm{(}}{{\rm{C}}_{\rm{8}}}{{\rm{H}}_{{\rm{18}}}}{\rm{)}}\;{\rm{ = }}\;{\rm{954}}\;{\rm{1000}}\;{\rm{ \times }}\;\frac{{{\rm{i}}\;{\rm{mol}}}}{{{\rm{114}}{\rm{.2}}\;{\rm{g}}}}\;{\rm{ = }}\;{\rm{viii}}{\rm{.35}}\;{\rm{mol}}\]
And now let'south see how many moles of O2 are needed to react with 8.35 mol of C8H18:
\[{\rm{n}}\;\left( {{{\rm{O}}_{\rm{two}}}} \correct)\;{\rm{ = }}\;{\rm{eight}}{\rm{.35 }}\cancel{{{\rm{mol}}\;{{\rm{C}}_{\rm{8}}}{{\rm{H}}_{{\rm{18}}}}}}\;{\rm{ \times }}\;\frac{{{\rm{25}}\;{\rm{mol}}\;{{\rm{O}}_{\rm{2}}}}}{{{\rm{ii}}\;\abolish{{{\rm{mol}}\;{{\rm{C}}_{\rm{viii}}}{{\rm{H}}_{{\rm{18}}}}}}}}\;{\rm{ = }}\;{\rm{104}}\;{\rm{mol}}\]
104 moles of Oii are needed to react with simply 8.35 mol of C8Heighteen and this is because of the 25:2 mole ratio. And then, 356 one thousand of oxygen is not plenty for the complete combustion of 954 k of octane.
xix.
When 140.0 g of AgNOiii was added to an aqueous solution of NaCl, 86.0 g of AgCl was collected every bit a white precipitate. Which salt was the limiting reactant in this reaction? How many grams of NaCl were present in the solution when AgNO3 was added?
AgNO3(aq) + NaCl(aq) → AgCl(south) + NaNOiii(aq)
answer
NaCl was the limiting reactant and 35.0 k of it were present in the solution when AgNOthreewas added.
Solution
We need to find the moles of AgCl which volition allow us to calculate how much AgNO3 and NaCl had reacted in the reaction.
\[{\rm{n}}\;{\rm{(AgCl)}}\;{\rm{ = }}\;{\rm{86}}{\rm{.0}}\;{\rm{g}}\;{\rm{ \times }}\;\frac{{{\rm{1}}\;{\rm{mol}}}}{{{\rm{143}}{\rm{.3}}\;{\rm{yard}}}}\;{\rm{ = }}\;{\rm{0}}{\rm{.600}}\;{\rm{mol}}\]
The stoichiometric ratio of all the reactants and products is1:ane, and therefore, there were 0.600 mol of AgNO3 and 0.600 mol of NaCl participating in the reaction.
Now, permit'south calculate the moles of 140.0 grand of AgNOiii and see whether it corresponds to 0.600 mol. If it is more than than that, then AgNOiii was added in backlog and NaCl must be the limiting reactant:
\[{\rm{n}}\;{\rm{(AgN}}{{\rm{O}}_{\rm{3}}}{\rm{)}}\;{\rm{ = }}\;{\rm{140}}{\rm{.0}}\;{\rm{g}}\;{\rm{ \times }}\;\frac{{{\rm{one}}\;{\rm{mol}}}}{{{\rm{169}}{\rm{.nine}}\;{\rm{m}}}}\;{\rm{ = }}\;{\rm{0}}{\rm{.824}}\;{\rm{mol}}\]
So, 0.824 mol of AgNO3 were added to the solution, just only 0.600 mol had reacted which ways that is how much NaCl was nowadays in the solution, and it was not enough to react with all the AgNO3.
And this confirms that AgNO3 was added in backlog and NaCl is the limiting reactant.
In the terminal step, nosotros make up one's mind the mass of 0.600 mol NaCl that was in the solution when AgNOthree was added:
\[{\rm{thousand}}\;{\rm{(NaCl)}}\;{\rm{ = }}\;{\rm{0}}{\rm{.600}}\;{\rm{mol}}\;{\rm{ \times }}\;\frac{{{\rm{58}}{\rm{.4}}\;{\rm{thousand}}}}{{{\rm{1}}\;{\rm{mol}}}}\;{\rm{ = }}\;{\rm{35}}{\rm{.0}}\;{\rm{g}}\]
20.
Consider the reaction between MnO2 and HCl:
MnOtwo + 4HCl → MnCltwo + Clii + 2HiiO
What is the theoretical yield of MnCl2 in grams when 165 m of MnOtwo is added to a solution containing 94.2 g of HCl?
answer
0.645 mol or 81.1 one thousand MnCl2
Solution
The theoretical yield is the amount of product that can be formed, so this problem is similar to the ones nosotros have been working on.
The amounts of both reactants are given, so we determine the LR and then, based on the moles of the LR, make up one's mind the amount of product(due south) that tin can exist formed.
The moles of MnOtwo and HCl are:
\[{\rm{northward}}\;{\rm{(Mn}}{{\rm{O}}_{\rm{two}}}{\rm{)}}\;{\rm{ = }}\;{\rm{165}}\;{\rm{g}}\;{\rm{ \times }}\;\frac{{{\rm{one}}\;{\rm{mol}}}}{{{\rm{86}}{\rm{.ix}}\;{\rm{m}}}}\;{\rm{ = }}\;{\rm{1}}{\rm{.xc}}\;{\rm{mol}}\]
\[{\rm{n}}\;{\rm{(HCl)}}\;{\rm{ = }}\;{\rm{94}}{\rm{.ii}}\;{\rm{g}}\;{\rm{ \times }}\;\frac{{{\rm{1}}\;{\rm{mol}}}}{{{\rm{36}}{\rm{.5}}\;{\rm{k}}}}\;{\rm{ = }}\;{\rm{2}}{\rm{.58}}\;{\rm{mol}}\]
Adjacent, we calculate how much MnClii tin can exist formed from each reactant:
\[{\rm{n}}\;\left( {{\rm{MnC}}{{\rm{fifty}}_{\rm{2}}}} \correct)\;{\rm{ = }}\;{\rm{1}}{\rm{.90 }}\cancel{{{\rm{mol}}\;{\rm{Mn}}{{\rm{O}}_{\rm{ii}}}}}\;{\rm{ \times }}\;\frac{{{\rm{i}}\;{\rm{mol}}\;{\rm{MnC}}{{\rm{fifty}}_{\rm{2}}}}}{{{\rm{1}}\;\cancel{{{\rm{mol}}\;{\rm{Mn}}{{\rm{O}}_{\rm{two}}}}}}}\;{\rm{ = }}\;{\rm{1}}{\rm{.90}}\;{\rm{mol}}\]
\[{\rm{north}}\;\left( {{\rm{MnC}}{{\rm{l}}_{\rm{2}}}} \right)\;{\rm{ = }}\;{\rm{ii}}{\rm{.58 }}\cancel{{{\rm{mol}}\;{\rm{HCl}}}}\;{\rm{ \times }}\;\frac{{{\rm{one}}\;{\rm{mol}}\;{\rm{MnC}}{{\rm{50}}_{\rm{two}}}}}{{{\rm{4}}\;\cancel{{{\rm{mol}}\;{\rm{HCl}}}}}}\;{\rm{ = }}\;{\rm{0}}{\rm{.645}}\;{\rm{mol}}\]
HCl gives less production so, information technology is the LR, and therefore, 0.645 mol MnCl2 is the theoretical yield of the reaction.
If you are asked to find the theoretical yield in grams, then catechumen the moles to grams using the molar mass of MnCl2:
\[{\rm{m}}\;{\rm{(MnC}}{{\rm{l}}_{\rm{2}}}{\rm{)}}\;{\rm{ = }}\;{\rm{0}}{\rm{.645}}\;{\rm{mol}}\;{\rm{ \times }}\;\frac{{{\rm{125}}{\rm{.eight}}\;{\rm{g}}}}{{{\rm{one}}\;{\rm{mol}}}}\;{\rm{ = }}\;{\rm{81}}{\rm{.1}}\;{\rm{chiliad}}\]
21.
Percent Yield
21. In a chemistry experiment, a student obtained 5.68 grand of a production. What is the pct yield of the product if the theoretical yield was 7.12 g?
answer
79.viii %
Solution
The percent yield of the reaction is the ratio of the actual over the theoretical yield. What we obtain is the actual yield and the maximum amount of product that can be obtained from the given corporeality of the limiting reactant is the theoretical yield.
Then, in this example, the actual yield is 5.68 one thousand, and the theoretical yield is 7.12 grand.
\[{\rm{\% }}\;{\rm{Yield}}\;{\rm{ = }}\;\frac{{\rm{A}}}{{\rm{T}}}\;{\rm{ = }}\;\frac{{{\rm{5}}{\rm{.68}}\;{\rm{thousand}}}}{{{\rm{7}}{\rm{.12}}\;{\rm{g}}}}\; \times \;100\% \; = \;79.8\;\% \]
22.
When 38.45 g CCliv is reacted with an excess of HF, 21.3 yard CCl2F2 is obtained. Summate the theoretical and percent yields of this reaction.
CCliv + 2HF → CCl2F2 + 2HCl
reply
70.five %
Solution
To notice the theoretical yield, we need to calculate the moles of CCl4 and, using the mole ratio, make up one's mind how much CCl2F2 can be formed.
\[{\rm{northward}}\;{\rm{(CC}}{{\rm{50}}_{\rm{four}}}{\rm{)}}\;{\rm{ = }}\;{\rm{38}}{\rm{.45}}\;{\rm{thou}}\;{\rm{ \times }}\;\frac{{{\rm{1}}\;{\rm{mol}}}}{{{\rm{153}}{\rm{.8}}\;{\rm{g}}}}\;{\rm{ = }}\;{\rm{0}}{\rm{.250}}\;{\rm{mol}}\]
The amount of CCl2F2 that can be formed from this is likewise 0.250 mol considering of the 1:1 tooth ratio:
\[{\rm{northward}}\;\left( {{\rm{CC}}{{\rm{l}}_{\rm{2}}}{{\rm{F}}_{\rm{ii}}}} \right)\;{\rm{ = }}\;{\rm{0}}{\rm{.25 }}\abolish{{{\rm{mol}}\;{\rm{CC}}{{\rm{50}}_{\rm{four}}}}}\;{\rm{ \times }}\;\frac{{{\rm{ane}}\;{\rm{mol}}\;{\rm{CC}}{{\rm{l}}_{\rm{2}}}{{\rm{F}}_{\rm{2}}}}}{{{\rm{i}}\;\cancel{{{\rm{mol}}\;{\rm{CC}}{{\rm{fifty}}_{\rm{4}}}}}}}\;{\rm{ = }}\;{\rm{0}}{\rm{.25}}\;{\rm{mol}}\]
The mass, which is also the theoretical yield of CCltwoF2 is:
\[{\rm{thousand}}\;{\rm{(CC}}{{\rm{l}}_{\rm{2}}}{{\rm{F}}_{\rm{2}}}{\rm{)}}\;{\rm{ = }}\;{\rm{0}}{\rm{.250}}\;{\rm{mol}}\;{\rm{ \times }}\;\frac{{{\rm{120}}{\rm{.9}}\;{\rm{g}}}}{{{\rm{i}}\;{\rm{mol}}}}\;{\rm{ = }}\;{\rm{30}}{\rm{.2}}\;{\rm{g}}\]
The percent yield of the reaction is the ratio of the actual over the theoretical yield:
\[{\rm{\% }}\;{\rm{Yield}}\;{\rm{ = }}\;\frac{{\rm{A}}}{{\rm{T}}}\;{\rm{ = }}\;\frac{{{\rm{21}}{\rm{.three}}\;{\rm{chiliad}}}}{{{\rm{30}}{\rm{.ii}}\;{\rm{yard}}}}\; \times \;100\% \; = \;70.5\;\% \]
23.
Atomic number 26(III) oxide reacts with carbon monoxide according to the equation:
Iron2O3(s) + 3CO(chiliad) → 2Fe(s) + 3COtwo(g)
What is the pct yield of this reaction if 623 thou of iron oxide produces 341 g of iron?
answer
78.iv%
Solution
The actual yield of the reaction is given equally 341 g Fe so, to determine the percent yield, we need to get-go calculate the theoretical yield. This is the amount of Fe that can be formed if all the starting textile (in this case Fe2Oiii) converts into a production according to the chemical equation.
Then, we are going to summate the moles of 623 g Fe2O3 and determine the moles and the mass of the atomic number 26 based on the stoichiometric ratio:
\[{\rm{due north}}\;{\rm{(F}}{{\rm{e}}_{\rm{2}}}{{\rm{O}}_{\rm{3}}}{\rm{)}}\;{\rm{ = }}\;{\rm{623}}\;{\rm{g}}\;{\rm{ \times }}\;\frac{{{\rm{one}}\;{\rm{mol}}}}{{{\rm{159}}{\rm{.7}}\;{\rm{g}}}}\;{\rm{ = }}\;{\rm{3}}{\rm{.xc}}\;{\rm{mol}}\]
The amount of Fethat tin can exist formed from this is calculated using the mole ratio of the oxide and iron:
\[{\rm{northward}}\;\left( {{\rm{Atomic number 26}}} \right)\;{\rm{ = }}\;{\rm{iii}}{\rm{.90 }}\cancel{{{\rm{mol}}\;{\rm{F}}{{\rm{e}}_{\rm{2}}}{{\rm{O}}_{\rm{iii}}}}}\;{\rm{ \times }}\;\frac{{{\rm{2}}\;{\rm{mol}}\;{\rm{Atomic number 26}}}}{{{\rm{i}}\;\abolish{{{\rm{mol}}\;{\rm{F}}{{\rm{e}}_{\rm{ii}}}{{\rm{O}}_{\rm{iii}}}}}}}\;{\rm{ = }}\;{\rm{seven}}{\rm{.80}}\;{\rm{mol}}\]
The mass, which is also the theoretical yield of Fe is:
\[{\rm{yard}}\;{\rm{(Fe)}}\;{\rm{ = }}\;{\rm{vii}}{\rm{.80}}\;{\rm{mol}}\;{\rm{ \times }}\;\frac{{{\rm{55}}{\rm{.viii}}\;{\rm{g}}}}{{{\rm{ane}}\;{\rm{mol}}}}\;{\rm{ = }}\;{\rm{435}}\;{\rm{g}}\]
The percent yield of the reaction is the ratio of the actual over the theoretical yield:
\[{\rm{\% }}\;{\rm{Yield}}\;{\rm{ = }}\;\frac{{\rm{A}}}{{\rm{T}}}\;{\rm{ = }}\;\frac{{{\rm{341}}\;{\rm{g}}}}{{{\rm{435}}\;{\rm{one thousand}}}}\; \times \;100\% \; = \;78.4\;\% \]
24.
Determine the percent yield of the reaction if 77.0 m of COii are formed from burning 2.00 moles of C5H12 in 4.00 moles of O2.
CvH12 + eight O2 → 5CO2 + 6H2O
answer
70.0 %
Solution
When the corporeality of both reactants is given, you lot must determine the LR and then, based on the moles of the LR, determine the amount of product(s) that can be formed. This would be the theoretical yield of COii.
At present, we are given the moles of the reactants, so we calculate how much CO2 can each produce:
\[{\rm{n}}\;\left( {{\rm{C}}{{\rm{O}}_{\rm{2}}}} \right)\;{\rm{ = }}\;{\rm{ii}}{\rm{.00 }}\cancel{{{\rm{mol}}\;{{\rm{C}}_{\rm{v}}}{{\rm{H}}_{{\rm{12}}}}}}\;{\rm{ \times }}\;\frac{{{\rm{5}}\;{\rm{mol}}\;{\rm{C}}{{\rm{O}}_{\rm{2}}}}}{{{\rm{1}}\;\cancel{{{\rm{mol}}\;{{\rm{C}}_{\rm{5}}}{{\rm{H}}_{{\rm{12}}}}}}}}\;{\rm{ = }}\;{\rm{10}}{\rm{.0}}\;{\rm{mol}}\]
\[{\rm{n}}\;\left( {{\rm{C}}{{\rm{O}}_{\rm{two}}}} \correct)\;{\rm{ = }}\;{\rm{4}}{\rm{.00 }}\cancel{{{\rm{mol}}\;{{\rm{O}}_{\rm{2}}}}}\;{\rm{ \times }}\;\frac{{{\rm{five}}\;{\rm{mol}}\;{\rm{C}}{{\rm{O}}_{\rm{2}}}}}{{{\rm{viii}}\;\abolish{{{\rm{mol}}\;{{\rm{O}}_{\rm{two}}}}}}}\;{\rm{ = }}\;{\rm{two}}{\rm{.50}}\;{\rm{mol}}\]
Because oxygen gives less product, it is the LR, and therefore, ii.50 mol COii could be formed in this reaction. This is the theoretical yield which is grams is:
\[{\rm{m}}\;{\rm{(C}}{{\rm{O}}_{\rm{ii}}}{\rm{)}}\;{\rm{ = }}\;{\rm{2}}{\rm{.50}}\;{\rm{mol}}\;{\rm{ \times }}\;\frac{{{\rm{44}}{\rm{.0}}\;{\rm{g}}}}{{{\rm{1}}\;{\rm{mol}}}}\;{\rm{ = }}\;{\rm{110}}{\rm{.}}\;{\rm{grand}}\]
The percent yield of the reaction is the ratio of the actual over the theoretical yield:
\[{\rm{\% }}\;{\rm{Yield}}\;{\rm{ = }}\;\frac{{\rm{A}}}{{\rm{T}}}\;{\rm{ = }}\;\frac{{{\rm{77}}{\rm{.0}}\;{\rm{g}}}}{{{\rm{110}}{\rm{.}}\;{\rm{thousand}}}}\; \times \;100\% \; = \;70.0\;\% \]
25.
The percentage yield for the following reaction was determined to exist 84%:
North2(grand) + 2Htwo(g) → N2Hfour(l)
How many grams of hydrazine (NorthiiH4) can exist produced when 38.36 chiliad of nitrogen reacts with six.68 g of hydrogen?
answer
36.8 g
Solution
The problem asks us to detect the actual yield of the reaction given that the per centum yield is lxxx%.
When the amounts of both reactants are given, y'all must make up one's mind the LR and and then, based on the moles of the LR, determine the corporeality of production(s) that can be formed. This would be the theoretical yield of hydrazine, and we tin can use it with the percentage yield to find the actual yield of the reaction.
\[{\rm{northward}}\;{\rm{(}}{{\rm{Northward}}_{\rm{ii}}}{\rm{)}}\;{\rm{ = }}\;{\rm{38}}{\rm{.36}}\;{\rm{g}}\;{\rm{ \times }}\;\frac{{{\rm{one}}\;{\rm{mol}}}}{{{\rm{28}}{\rm{.0}}\;{\rm{m}}}}\;{\rm{ = }}\;{\rm{1}}{\rm{.37}}\;{\rm{mol}}\]
\[{\rm{n}}\;{\rm{(}}{{\rm{H}}_{\rm{ii}}}{\rm{)}}\;{\rm{ = }}\;{\rm{half dozen}}{\rm{.68}}\;{\rm{grand}}\;{\rm{ \times }}\;\frac{{{\rm{one}}\;{\rm{mol}}}}{{{\rm{two}}{\rm{.00}}\;{\rm{chiliad}}}}\;{\rm{ = }}\;{\rm{3}}{\rm{.34}}\;{\rm{mol}}\]
At present, we are given the moles of the reactants, so we calculate how much N2Hiv can each produce:
\[{\rm{n}}\;\left( {{{\rm{Northward}}_{\rm{2}}}{{\rm{H}}_{\rm{iv}}}} \right)\;{\rm{ = }}\;{\rm{ane}}{\rm{.37 }}\cancel{{{\rm{mol}}\;{{\rm{N}}_{\rm{two}}}}}\;{\rm{ \times }}\;\frac{{{\rm{1}}\;{\rm{mol}}\;{{\rm{N}}_{\rm{2}}}{{\rm{H}}_{\rm{4}}}}}{{{\rm{1}}\;\abolish{{{\rm{mol}}\;{{\rm{N}}_{\rm{2}}}}}}}\;{\rm{ = }}\;{\rm{ane}}{\rm{.37}}\;{\rm{mol}}\]
\[{\rm{north}}\;\left( {{{\rm{N}}_{\rm{2}}}{{\rm{H}}_{\rm{4}}}} \correct)\;{\rm{ = }}\;{\rm{3}}{\rm{.34 }}\cancel{{{\rm{mol}}\;{{\rm{H}}_{\rm{2}}}}}\;{\rm{ \times }}\;\frac{{{\rm{1}}\;{\rm{mol}}\;{{\rm{N}}_{\rm{2}}}{{\rm{H}}_{\rm{four}}}}}{{{\rm{ii}}\;\cancel{{{\rm{mol}}\;{{\rm{H}}_{\rm{2}}}}}}}\;{\rm{ = }}\;{\rm{i}}{\rm{.67}}\;{\rm{mol}}\]
Northwardtwo gives less hydrazine and so, it is the LR, and therefore the theoretical yield of N2Hfour is 1.37 mol. The mass of Due north2H4 is:
\[{\rm{one thousand}}\;{\rm{(}}{{\rm{N}}_{\rm{2}}}{{\rm{H}}_{\rm{four}}}_{\rm{2}}{\rm{)}}\;{\rm{ = }}\;{\rm{1}}{\rm{.37}}\;{\rm{mol}}\;{\rm{ \times }}\;\frac{{{\rm{32}}{\rm{.0}}\;{\rm{chiliad}}}}{{{\rm{one}}\;{\rm{mol}}}}\;{\rm{ = }}\;{\rm{43}}{\rm{.8}}\;{\rm{m}}\]
The percent yield of the reaction is the ratio of the actual over the theoretical yield, and then rearranging this equation we can summate the actual yield:
\[{\rm{\% }}\;{\rm{Yield}}\;{\rm{ = }}\;\frac{{\rm{A}}}{{\rm{T}}}\;\]
A = T 10 % Yield
A = 43.8 x 0.84 = 36.viii thou
Some other approach for determining the actual yield is to convert the moles of limiting reactant to the percentage of the percent yield. And then, if the yield is 84%, we could have multiplied the moles of Nii by 0.84 and determine the mass of NtwoHiv based on that.
Nosotros would have 1.37 mole x 0.84 = 1.1508 mol N2 which is the moles of nitrogen that actually convert into hydrazine. By the mole ratio, the moles of N2H4 would exist:
\[{\rm{n}}\;\left( {{{\rm{N}}_{\rm{2}}}{{\rm{H}}_{\rm{4}}}} \correct)\;{\rm{ = }}\;{\rm{i}}{\rm{.1508 }}\abolish{{{\rm{mol}}\;{{\rm{N}}_{\rm{2}}}}}\;{\rm{ \times }}\;\frac{{{\rm{1}}\;{\rm{mol}}\;{{\rm{Due north}}_{\rm{two}}}{{\rm{H}}_{\rm{iv}}}}}{{{\rm{one}}\;\cancel{{{\rm{mol}}\;{{\rm{N}}_{\rm{two}}}}}}}\;{\rm{ = }}\;{\rm{one}}{\rm{.1508}}\;{\rm{mol}}\]
And the mass would be:
\[{\rm{m}}\;{\rm{(}}{{\rm{Due north}}_{\rm{2}}}{{\rm{H}}_{\rm{four}}}_{\rm{2}}{\rm{)}}\;{\rm{ = }}\;{\rm{1}}{\rm{.1508}}\;{\rm{mol}}\;{\rm{ \times }}\;\frac{{{\rm{32}}{\rm{.0}}\;{\rm{1000}}}}{{{\rm{i}}\;{\rm{mol}}}}\;{\rm{ = }}\;{\rm{36}}{\rm{.8}}\;{\rm{k}}\]
26.
Silver metallic can exist prepared by reducing its nitrate, AgNO3 with copper co-ordinate to the post-obit equation:
Cu(due south) + 2AgNO3(aq) → Cu(NO3)two(aq) + 2Ag(southward)
What is the pct yield of the reaction if 71.v grams of Ag was obtained from 132.5 grams of AgNO3?
answer
85%
Solution
The actual yield of the reaction is given as 71.5 g Ag so, to determine the percent yield, we demand to first calculate the theoretical yield. This is the amount of Ag that can exist formed if all the starting cloth (in this example AgNO3) converts into product according to the chemical equation.
So, we are going to calculate the moles of 132.5 one thousand AgNO3 and determine the moles and the mass of the silver based on their stoichiometric ratio:
\[{\rm{n}}\;{\rm{(AgN}}{{\rm{O}}_{\rm{3}}}{\rm{)}}\;{\rm{ = }}\;{\rm{132}}{\rm{.five}}\;{\rm{thou}}\;{\rm{ \times }}\;\frac{{{\rm{i}}\;{\rm{mol}}}}{{{\rm{169}}{\rm{.9}}\;{\rm{g}}}}\;{\rm{ = }}\;{\rm{0}}{\rm{.780}}\;{\rm{mol}}\]
\[{\rm{north}}\;\left( {{\rm{Ag}}} \correct)\;{\rm{ = }}\;{\rm{0}}{\rm{.780 }}\cancel{{{\rm{mol}}\;{\rm{AgN}}{{\rm{O}}_{\rm{3}}}}}\;{\rm{ \times }}\;\frac{{{\rm{2}}\;{\rm{mol}}\;{\rm{Ag}}}}{{{\rm{two}}\;\abolish{{{\rm{mol}}\;{\rm{AgN}}{{\rm{O}}_{\rm{3}}}}}}}\;{\rm{ = }}\;{\rm{0}}{\rm{.780}}\;{\rm{mol}}\]
The mass, which is also the theoretical yield of Ag is:
\[{\rm{thousand}}\;{\rm{(Ag)}}\;{\rm{ = }}\;{\rm{0}}{\rm{.780}}\;{\rm{mol}}\;{\rm{ \times }}\;\frac{{{\rm{107}}{\rm{.nine}}\;{\rm{g}}}}{{{\rm{1}}\;{\rm{mol}}}}\;{\rm{ = }}\;{\rm{84}}{\rm{.2}}\;{\rm{m}}\]
The percent yield of the reaction is the ratio of the actual over the theoretical yield:
\[{\rm{\% }}\;{\rm{Yield}}\;{\rm{ = }}\;\frac{{\rm{A}}}{{\rm{T}}}\;{\rm{ = }}\;\frac{{{\rm{71}}{\rm{.5}}\;{\rm{thousand}}}}{{{\rm{84}}{\rm{.2}}\;{\rm{one thousand}}}}\; \times \;100\% \; = \;85\;\% \]
27.
Industrially, nitric acid is produced from ammonia by the Ostwald process in a series of reactions:
4NH3(g) + 5O2(g) → 4NO(g) + 6HtwoO(l)
2NO(g) + Oii(grand) → 2NO2(g)
2NO2(g) + H2O(fifty) → HNO3(aq) + HNOtwo(aq)
Considering that each reaction has an 85% percent yield, how many grams of NH3 must be used to produce 25.0 kg of HNO3 by the above procedure?
answer
2.xx 10 104 grand
Solution
Because we are given the mass of the product and need to determine the mass of the starting material, the calculations are going to exist in reverse gild. So, first, nosotros can calculate the moles of HNOthree and using the mole ratio, discover the moles of NO2 which is the link between the 3rd and 2nd reactions.
Earlier using the mass of HNO3, catechumen it to grams because the molar mass is given in grams per mole:
\[{\rm{m}}\left( {{\rm{HN}}{{\rm{O}}_{\rm{3}}}} \right)\;{\rm{ = }}\;{\rm{25}}{\rm{.0}}\;\cancel{{{\rm{kg}}}}\;{\rm{ \times }}\;\frac{{{\rm{1000}}\;{\rm{g}}}}{{{\rm{1}}\;\abolish{{{\rm{kg}}}}}}\;{\rm{ = }}\;{\rm{2}}{\rm{.5}}\;{\rm{ \times }}\;{\rm{ane}}{{\rm{0}}^{\rm{4}}}\;{\rm{g}}\]
\[{\rm{north}}\;{\rm{(HN}}{{\rm{O}}_{\rm{iii}}}{\rm{)}}\;{\rm{ = }}\;{\rm{2}}{\rm{.fifty}}\;{\rm{ \times }}\;{10^4}\;{\rm{yard}}\;{\rm{ \times }}\;\frac{{{\rm{one}}\;{\rm{mol}}}}{{{\rm{63}}{\rm{.0}}\;{\rm{g}}}}\;{\rm{ = }}\;{\rm{397}}\;{\rm{mol}}\]
\[{\rm{n}}\;\left( {{\rm{N}}{{\rm{O}}_{\rm{2}}}} \right)\;{\rm{ = }}\;{\rm{397 }}\cancel{{{\rm{mol}}\;{\rm{HN}}{{\rm{O}}_{\rm{3}}}}}\;{\rm{ \times }}\;\frac{{{\rm{2}}\;{\rm{mol}}\;{\rm{N}}{{\rm{O}}_{\rm{ii}}}}}{{{\rm{i}}\;\abolish{{{\rm{mol}}\;{\rm{HN}}{{\rm{O}}_{\rm{3}}}}}}}\;{\rm{ = }}\;{\rm{794}}\;{\rm{mol}}\]
Now, this would have been the conversion if it was a process with a 100% yield. However, considering each step of this procedure has an 85% percentage yield, we need to multiply each mole conversion past a factor of
\[\frac{{{\rm{100\% }}}}{{{\rm{85\% }}}}\;{\rm{or}}\;{\rm{simply}}\;\frac{{{\rm{100}}}}{{{\rm{85}}}}\]
And then, the moles of NO2 that produced 397 mol of HNOiii would be:
\[{\rm{northward}}\;\left( {{\rm{N}}{{\rm{O}}_{\rm{ii}}}} \correct)\;{\rm{ = }}\;{\rm{794}}\;{\rm{mol}}\;{\rm{ \times }}\;\frac{{{\rm{100}}}}{{{\rm{85}}}}\;{\rm{ = }}\;{\rm{934}}\;{\rm{mol}}\]
Now, we go to the second reaction and see how much NO was needed to produce 934 mol NOtwo because that the yield of the reaction is again 85%.
2NO(one thousand) + O2(yard) → 2NOtwo(yard)
\[{\rm{northward}}\;\left( {{\rm{NO}}} \right)\;{\rm{ = }}\;{\rm{934 }}\cancel{{{\rm{mol}}\;{\rm{North}}{{\rm{O}}_{\rm{ii}}}}}\;{\rm{ \times }}\;\frac{{{\rm{two}}\;{\rm{mol}}\;{\rm{NO}}}}{{{\rm{ii}}\;\cancel{{{\rm{mol}}\;{\rm{N}}{{\rm{O}}_{\rm{2}}}}}}}\; \times \;\frac{{100}}{{85}}\;{\rm{ = }}\;{\rm{1098}}\;{\rm{mol}}\]
Side by side, we do the same for the first reaction and determine how much NH3 was needed to produce 1098 mol NO considering that the yield of the reaction is again 85%.
4NH3(g) + 5O2(g) → 4NO(one thousand) + 6HiiO(fifty)
\[{\rm{n}}\;\left( {{\rm{N}}{{\rm{H}}_{\rm{iii}}}} \right)\;{\rm{ = }}\;{\rm{1098 }}\cancel{{{\rm{mol}}\;{\rm{NO}}}}\;{\rm{ \times }}\;\frac{{{\rm{4}}\;{\rm{mol}}\;{\rm{N}}{{\rm{H}}_{\rm{iii}}}}}{{{\rm{4}}\;\cancel{{{\rm{mol}}\;{\rm{NO}}}}}}\;{\rm{ \times }}\;\frac{{{\rm{100}}}}{{{\rm{85}}}}\;{\rm{ = }}\;{\rm{1293}}\;{\rm{mol}}\]
In the final step, we convert the moles to the mass of ammonia:
\[{\rm{m}}\;{\rm{(N}}{{\rm{H}}_{\rm{3}}}{\rm{)}}\;{\rm{ = }}\;{\rm{1293}}\;{\rm{mol}}\;{\rm{ \times }}\;\frac{{{\rm{17}}{\rm{.0}}\;{\rm{thou}}}}{{{\rm{1}}\;{\rm{mol}}}}\;{\rm{ = }}\;{\rm{21,981}}\;{\rm{g}}\]
Rounding off to three meaning figures, nosotros get:
2.20 x xfour g
28.
Aspirin (acetyl salicylic acid) is widely used to treat pain, fever, and inflammation. It is produced from the reaction of salicylic acid with acerb anhydride. The chemical equation for aspirin synthesis is shown below:
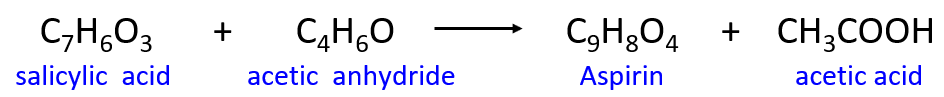
In one container, 10.00 kg of salicylic acrid is mixed with 10.00 kg of acerb anhydride.
a) Which reactant is limiting? Which is in excess?
b) What mass of backlog reactant is left over?
c) What mass of aspirin is formed assuming 100% yield (Theoretical yield)?
d) What mass of aspirin is formed if the reaction yield is 70.0% ?
e) If the actual yield of aspirin is 11.2 kg, what is the percent yield?
f) How many kg of salicylic acrid is needed to produce 20.0 kg of aspirin if the reaction yield is 85.0% ?
a)
respond
Salicylic acid is the limiting reagent
b)
answer
2.608 x 103 g Acetic anhydride
c)
answer
1.305 x ten4 thousand Aspirin
d)
answer
9.14 x 103 thousand Aspirin
e)
answer
85.viii %
f)
answer
18.one kg
Solution
a)Which reactant is limiting? Which is in excess?
The limiting reactant is the one producing less production, then nosotros need to calculate the moles of 00 kg salicylic acid and x.00 kg acerb anhydride and meet which 1 produces less aspirin. Because the mole ratio of all the reactants and products is 1:1, whichever has a smaller number of moles, it is the limiting reactant.
Earlier using the masses, catechumen them to grams because the tooth mass is given in grams per mole:
\[{\rm{m}}\left( {{\rm{salicylic acid}}} \right)\;{\rm{ = }}\;{\rm{10}}{\rm{.00}}\;\cancel{{{\rm{kg}}}}\;{\rm{ \times }}\;\frac{{{\rm{1000}}\;{\rm{g}}}}{{{\rm{1}}\;\cancel{{{\rm{kg}}}}}}\;{\rm{ = }}\;{\rm{i}}{\rm{.000}}\;{\rm{ \times }}\;{\rm{i}}{{\rm{0}}^{\rm{4}}}\;{\rm{g}}\]
\[{\rm{k}}\left( {{\rm{acetic anhydride}}} \right)\;{\rm{ = }}\;{\rm{x}}{\rm{.00}}\;\abolish{{{\rm{kg}}}}\;{\rm{ \times }}\;\frac{{{\rm{1000}}\;{\rm{g}}}}{{{\rm{1}}\;\abolish{{{\rm{kg}}}}}}\;{\rm{ = }}\;{\rm{1}}{\rm{.000}}\;{\rm{ \times }}\;{\rm{one}}{{\rm{0}}^{\rm{4}}}\;{\rm{g}}\]
\[{\rm{north}}\;{\rm{(salicylic acid)}}\;{\rm{ = }}\;{\rm{one}}{\rm{.000}}\;{\rm{ \times }}\;{\rm{1}}{{\rm{0}}^{\rm{4}}}\;{\rm{one thousand}}\;{\rm{ \times }}\;\frac{{{\rm{ane}}\;{\rm{mol}}}}{{{\rm{138}}{\rm{.1}}\;{\rm{grand}}}}\;{\rm{ = }}\;{\rm{72}}{\rm{.4}}\;{\rm{mol}}\]
\[{\rm{due north}}\;{\rm{(acerb anhydride)}}\;{\rm{ = }}\;{\rm{1}}{\rm{.000}}\;{\rm{ \times }}\;{\rm{1}}{{\rm{0}}^{\rm{4}}}\;{\rm{one thousand}}\;{\rm{ \times }}\;\frac{{{\rm{1}}\;{\rm{mol}}}}{{{\rm{102}}{\rm{.1}}\;{\rm{thousand}}}}\;{\rm{ = }}\;{\rm{97}}{\rm{.9}}\;{\rm{mol}}\]
Salicylic acid is present in a smaller quantity, and because of the 1:i molar ratio of all the reactants and products, it is going to produce less aspirin, and therefore, it is the limiting reactant.
b) What mass of excess reactant is left over?
We know that salicylic acid is the limiting reactant, so the calculations are going to be based on its moles (72.4 mol). Using the mole ratio, we can calculate how much acetic anhydride has reacted. The remaining mass of the acetic anhydride is calculated by subtracting this corporeality from the initial mass.
Again, because of the one:1 molar ratio, there is going to be 72.4 mol acetic anhydride reacted:
\[{\rm{n}}\;\left( {{\rm{acetic anhydride}}} \correct)\;{\rm{ = }}\;{\rm{72}}{\rm{.4 }}\cancel{{{\rm{mol}}\;{\rm{salicylic acid}}}}\;{\rm{ \times }}\;\frac{{{\rm{1}}\;{\rm{mol}}\;{\rm{acetic anhydride}}}}{{{\rm{1}}\;\cancel{{{\rm{mol}}\;{\rm{salicylic acrid}}}}}}\;{\rm{ = }}\;{\rm{72}}{\rm{.4}}\;{\rm{mol}}\]
This corresponds to:
\[{\rm{chiliad}}\;{\rm{(acetic anhydride)}}\;{\rm{ = }}\;{\rm{72}}{\rm{.4}}\;{\rm{mol}}\;{\rm{ \times }}\;\frac{{{\rm{102}}{\rm{.i}}\;{\rm{g}}\;}}{{{\rm{1}}\;{\rm{mol}}}}\;{\rm{ = }}\;{\rm{7392}}\;{\rm{one thousand}}\]
The remaining mass of acetic anhydride is:
10,000 – 7392 = 2608 g = ii.608 kg
c) What mass of aspirin is formed assuming 100% yield (Theoretical yield)?
72.iv mol aspirin will exist formed considering of the ane:i molar ratio:
\[{\rm{n}}\;\left( {{\rm{aspirin}}} \right)\;{\rm{ = }}\;{\rm{72}}{\rm{.4 }}\cancel{{{\rm{mol}}\;{\rm{salicylic acrid}}}}\;{\rm{ \times }}\;\frac{{{\rm{one}}\;{\rm{mol}}\;{\rm{aspirin}}}}{{{\rm{1}}\;\abolish{{{\rm{mol}}\;{\rm{salicylic acid}}}}}}\;{\rm{ = }}\;{\rm{72}}{\rm{.4}}\;{\rm{mol}}\]
Using the molar mass of aspirin, we tin summate its mass for 100%-yield reaction:
\[{\rm{m}}\;{\rm{(aspirin)}}\;{\rm{ = }}\;{\rm{72}}{\rm{.4}}\;{\rm{mol}}\;{\rm{ \times }}\;\frac{{{\rm{180}}{\rm{.2}}\;{\rm{k}}}}{{{\rm{ane}}\;{\rm{mol}}}}\;{\rm{ = }}\;{\rm{xiii,046}}\;{\rm{g}}\]
This corresponds to 13.05 kg when divided by 1000.
d) What mass of aspirin is formed if the reaction yield is 70.0%?
To find the mass of aspirin when the percent yield is 70.0%, nosotros need to multiply the theoretical yield (13.05 kg) by 0.700:
1000 (aspirin) = 13.05 kg 10 0.700 = 9.14 kg
e) If the actual yield of aspirin is 11.2 kg, what is the percentage yield?
The percent yield of the reaction is the ratio of the bodily over the theoretical yield:
\[{\rm{\% }}\;{\rm{Yield}}\;{\rm{ = }}\;\frac{{\rm{A}}}{{\rm{T}}}\;{\rm{ = }}\;\frac{{{\rm{xi}}{\rm{.2}}\;{\rm{kg}}}}{{{\rm{13}}{\rm{.05}}\;{\rm{kg}}}}\;{\rm{ \times }}\;{\rm{100\% }}\;{\rm{ = }}\;{\rm{85}}{\rm{.8}}\;{\rm{\% }}\]
f) How many kg of salicylic acid is needed to produce 20.0 kg of aspirin if the reaction yield is 85.0% ?
Offset, calculate the moles of 20.0 kg aspirin which is 2.00 ten 10four g:
\[{\rm{north}}\;{\rm{(aspirin)}}\;{\rm{ = }}\;{\rm{two}}{\rm{.0}}\;{\rm{ \times }}\;{\rm{one}}{{\rm{0}}^{\rm{four}}}\;{\rm{one thousand}}\;{\rm{ \times }}\;\frac{{{\rm{one}}\;{\rm{mol}}}}{{{\rm{180}}{\rm{.2}}\;{\rm{g}}}}\;{\rm{ = }}\;{\rm{111}}\;{\rm{mol}}\]
According to the mole ratio of the chemic equation, this is how much salicylic acid would exist needed because of the 1:ane mole ratio.
However, considering the reaction has an 85.0% pct yield, we need to multiply the moles by a gene of
\[{\rm{n}}\;{\rm{(salicylic acid)}}\;{\rm{ = }}\;{\rm{111}}\;{\rm{mol}}\;{\rm{ \times }}\;\frac{{{\rm{100}}}}{{{\rm{85}}}}\;{\rm{ = }}\;{\rm{131}}\;{\rm{mol}}\]
The mass of salicylic acid would be:
\[{\rm{1000}}\;{\rm{(salicylic acid)}}\;{\rm{ = }}\;{\rm{131}}\;{\rm{mol}}\;{\rm{ \times }}\;\frac{{{\rm{138}}\;{\rm{g}}}}{{{\rm{ane}}\;{\rm{mol}}}}\;{\rm{ = }}\;{\rm{18,078}}\;{\rm{yard}}\]
This corresponds to 1.81 kg
Stoichiometry Worksheet 1 Mass Mass,
Source: https://general.chemistrysteps.com/stoichiometry-practice-problems/
Posted by: plumbupasylat.blogspot.com


0 Response to "Stoichiometry Worksheet 1 Mass Mass"
Post a Comment